|
Taxonomic
History:
Acanthocyclops vernalis can be commonly
misidentified in a sample as the morphologically similar Cyclops vernalis, C. americanus, or even C. brevispinosus.
Originally a subgenus under genus Cyclops, Kiefer (1960) noted
there are actually several genera that fall under Cyclops, hence the reason Acanthocyclops is now its own genus with over 70
species/subspecies (Dussart and Defaye,
2006). The work of Kiefer (1978) indicated that A. robustus
found in the Great Lakes may be a new
species (possibly arising due to different environmental pressures), being
regularly misidentified as A. vernalis.
Though a key does exist to distinguish A. vernalis
from A. robustus (Dodson, 1994), the
characteristics it uses for separation are often difficult to detect. Dodson
et al. (2003) noted that these irregularly present characteristics
(Especially: a patch of spines on the anterior face of the P4 coxa and two terminal spines on the terminal segment of
the of the P4 endopod.) are due to cryptic
speciation – a situation where populations become genetically distinct but
maintain morphological similarity. A. robustus
is still commonly, and acceptingly, identified as A. vernalis
since morphologic characterization is so unpredictable (Balcer
et al., 1984).
Anatomy:
A. vernalis exhibit sexual
dimorphism, with males being smaller – Length: 0.8-1.0 mm. Dry weight:
2.4-2.6g – than females – Length: 1.0-1.4 mm. Dry weight: 4.8-6.4g – (see Fig. 1) (Balcer et al., 1984; Hawkins and Evans, 1979). Figure 2
shows the four terminal setae per caudal ramus. The medial pair of setae are longest and the lateral seta is always found along the
caudal ramus within one third of the posterior end (Fig. 2).
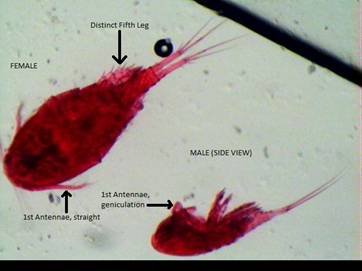
Fig. 1: Acanthocyclops
vernalis female (left) and male (right) showing
sexual dimorphic size, 1st antennae geniculation in males, and the
distinct 5th leg, often useful in distinguishing A. vernalis from Diacyclops
thomasi (Balcer et al.,
1984).
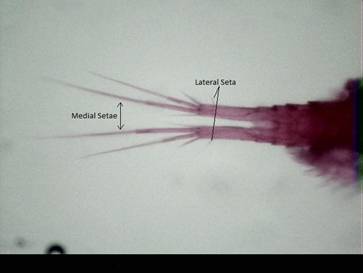
Fig. 2: A. vernalis’ tail showing elongate medial setae and
lateral seta located within one third the distance down the posterior end of
the caudal ramus.
Like all copepods in subclass Copepoda, A. vernalis
lack a compound eye. Both first antenna of the male are geniculate (Fig. 1
and Fig. 3) while the female’s are straight; none usually reaching past the
genital segment. Though similar in many ways to Diacyclops
thomasi, A. vernalis’
5th legs are distinct (Balcer et al.,
1984).
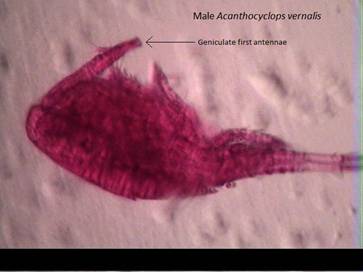
Fig. 3: A. vernalis
male showing the geniculate 1st antenna present on both antennae.
|
Distribution:
A. vernalis are found in all
five Great Lakes (Balcer
et al., 1984). And, though one of the most common species in North America (Yeatman,
1944), Patalas (1972) reports that they only make
up less than 1% of the crustacean zooplankton in our Great
Lakes. The higher the eutrophic level of the lake, the more likely
there will be a healthy population of A. vernalis present (Balcer
et al., 1984).
Habitat:
Fryer (1985) states that A. vernalis in Europe
are “exclusively benthic organisms”, preferring lightly acidic waters low in
calcium and total ion concentration. Evans and Stewart (1977) would agree
that A. vernalis are mainly benthic, but
they can be found throughout the water column (thus epibenthic),
possibly even exhibiting diurnal migration – coming up to the top of the
water column at night. Their centralization to nearshore
areas in mainly eutrophic lake conditions potentially makes Acanthocyclops vernalis
a good indicator of lake ecosystem health. For open water, Lake
Erie’s western basin seems to be the only place in the Great Lakes region eutrophic enough for these organisms
to live (Patalas, 1972).
Feeding:
These predacious organisms are known to
consume Bosmina, Ceriodaphnia
reticulata, cladocerans,
and even their own nauplii (Balcer
et al., 1984). The smallest prey item is not always the one that is chosen
for food. Carapace integrity, shape, and escape strategy seem to be the three
most important factors to a prey item if it wishes to survive (Li and Li,
1979). Li and Li (1979) list Asplanchna, Diaphanosoma, and Diaptomus
as the three preferred species of A. vernalis.
For bacteria, there is still a fair amount of debate over whether or not
copepods in general are bactivores (Work and
Havens, 2003).
Life
History:
A. vernalis is able to reproduce
throughout the year in some lakes, even under the ice. However, reproduction
is highly dependant upon temperature, where extremely high temperatures cause
dormancy and low temperatures will slow reproduction. Predictably, when
temperatures are favorable (20oC), A. vernalis
produce many small offspring (50% mortality, 7-8 days to mature) but when
temperatures drop to 7-10oC, the adults produce larger, fewer
offspring at a slower rate (92% mortality, 44 days to mature). In Great Lakes studies, few to no A. vernalis are present in samples taken between
December and May. There is an 8-10oC cutoff observed in Lake Erie, below which A. vernalis
adults are not commonly seen. And, Lake Superior
exhibits the slowest reproduction, producing just one generation each year (Balcer et al., 1984).
In the absolute optimal conditions, the
female will hatch, on average, a brood every 36 hours for up to four weeks.
After one mating event, 40-80 eggs are dropped into the two egg sacs.
However, in the case of Lake Superior, diapausing copepodids, stuck in
the CIV or CV stage, are important for continuing the population after they
hatch the next spring in more optimal temperatures (Balcer
et al., 1984).
|
Works Cited:
Balcer, M.D., Korda,
N.L., Dodson, S.I. (1984). Zooplankton of the Great Lakes; A guide to the Identification and Ecology
of the Common Crustacean Species. The University
of Wisconson Press, 93-95.
Dodson, S. (1994). Morphological Analysis of
Wisconsin (U.S.A.) Species of the Acanthocyclops
vernalis Group (Copepoda:
Cyclopoida). Journal of Crustacean Biology,
14(1), 113-131.
Dodson, S.I., Grishanin,
A.K., Gross, K, Wyngaard, G.A. (2003). Morphological analysis of some cryptic species in the Acanthocyclops vernalis
species complex from North America. Hydrobiologia, 500, 131-143.
Dussart, B.H. and Defaye, D. (2006). World Directory of Crustacea Copepoda. II-Cyclopiformes. Backhuys Publishers, Leiden.
Evans, M.S. and Stewart, J.A.
(1977). Epibenthic and
Benthic microcrustaceans (copepods, cladocerans, ostracods) from a nearshore area in southeastern Lake
Michigan. Limnology and Oceanography, 22(6),
1059-1066.
Fryer, G. (1985). An ecological validation of a
taxonomic distinction: the ecology of Acanthocyclops
vernalis and A. robustus
(Crustacea: Gopepoda). Zoological
Journal of the Linnean Society, 84(2), 165-180.
Hawkins, B.E. and Evans, M.S.
(1979).
Seasonal cycles of zooplankton biomass in southeastern Lake
Michigan. Journal of Great Lakes
Research, 5(3-4), 256-263.
Kiefer, F. (1960). Ruderfuskrebse
(Copepoda). Kosmos-Verlag, Stuttgart.
Kiefer, F. (1978). Freilebende
Copepoda. Binnengewasser,
26(2): 1-343.
Li, J.L. and Li, H.W. (1979). Species-Specific Factors
Affecting Predator-Prey Interactions of the Copepod Acanthocyclops
vernalis with its Natural Prey. Limnology
and Oceanography, 24(4), 613-626.
Patalas, K. (1972). Crustacean
plankton and the eutrophication of St. Lawrence Great Lakes. Journal
of the Fisheries Research Board Canada, 29(10), 1451-1462.
Work, K.A. and Havens, K.E.
(2003). Short Communication; Zooplankton grazing on bacteria and
cyanobacteria in a eutrophic lake. Journal of Plankton Research,
25(10), 1301-1307.
Yeatman, H.C. (1944). American cyclopoid copepods of the viridis-vernalis
group (including a description of Cyclops carolinianus
n.sp.). American Midland
Naturalist, 32(1), 1-90.
Publishers.
|