|
Site
created by: Margaret Van Guilder Organism: Moina micrura
|
|
Taxonomic Classification: Kingdom:
Animalia Phylum:
Arthropoda Sub-phylum:
Crustacea Class:
Branchiopoda Order:
Cladocera Sub-order:
Eucladocera Family:
Moinidae Genus:
Moina Species:
Moina micrura The cosmopolitan Moina
micrura is argued to be not a single species, but rather a cryptic
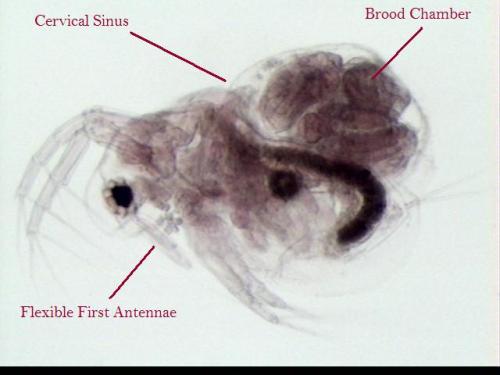
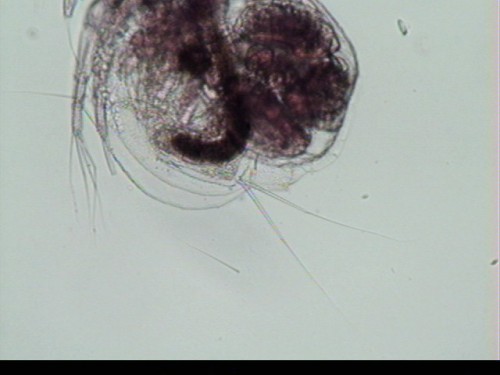
species complex (Martínez-Jerónimo et al., 2007). Anatomy: Approximately 0.5 mm in length, Moina micrura (Figure 1) is relatively
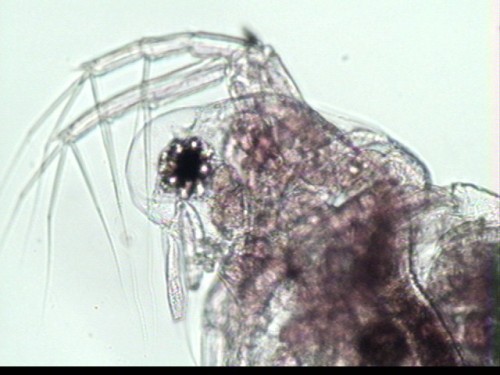
rounded in body shape, yet possesses a relatively large, distinct head
(Balcer et al., 1984). The head is
approximately ½ the length of the body and is curved or sloped ventrally
(Figure 2). The first antennae are
exposed (not covered by a beak), variable in length, flexible and are attached
along the ventral surface of the head, rather than at the front (Figure
1). The tips of the antennae are blunt
and exhibit short olfactory setae. The
second antennae are large and used for swimming. The body is surrounded by a shell-like
carapace which is open along the ventral surface and includes a notch-like
cervical sinus (near the “nape”) on the dorsal surface of the body. The dorsal surface also includes a brood
chamber to carry eggs (Figure 1). Moina micrura lacks a rear shell
spine, rostrum and ocellus, or eye spot, though does exhibit a single large,
median compound eye. M. micrura does exhibit a
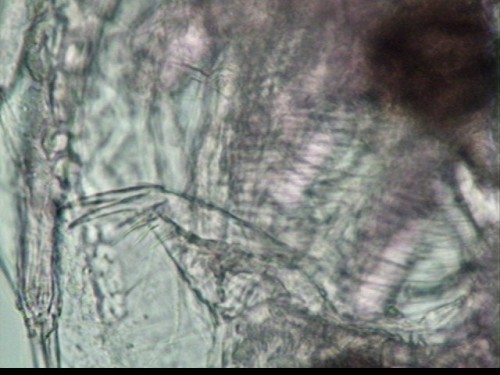
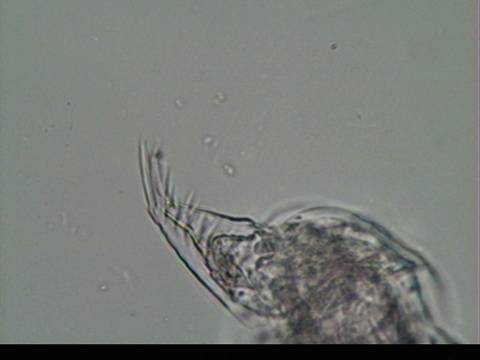
post-abdominal claw with pecten of uniform length (Figures 3 and 4). Dorsal to the postabdomen are two pairs of
relatively long abdominal setae (Figure 5) (Balcer et al., 1984). Distribution and
Habitat: Moina micrura is distributed across the globe. Samples have been collected from North
America (Balcer et al., 1984; Martínez-Jerónimo et al., 2007), Europe
(Crosetti and Margaritora, 1987), South America (Fileto et al., 2004; Fileto
et al., 2010), Africa (Hart, 1990) and Southeast Asia (Jana and Pal,
1985). M. micrura is rarely found inhabiting the Great Lakes, though it
has been found in Lake Michigan near Green Bay (Balcer et al., 1984). It has been shown to inhabit temporary
pools that are often highly eutrophic and have shallow depth (Crosetti and
Margaritora, 1987). Relatively turbid
lakes high in nutrients tend to encourage M.
micrura habitation (Hart, 1990; Jana and Pal, 1985). Feeding Ecology: Moina micrura are filtering grazers of small phytoplankton (Balcer et al.,
1984). Feeding occurs when water is
moved across the thoracic appendages.
Food particles in the moving water are often brought into the
carapace. Any floating phytoplankton
is trapped by the setae on the thoracic legs and then moved to the mouth to
be consumed. Food particles are most
often algae, yet can also consist of bacteria, protozoa and organic detritus
(Balcer et al., 1984). Food particles
are often selected based on size (primarily), shape, chemical cues, taste and
nutritional content (Pagano, 2008).
Fileto et al. (2004) determined that very small and very large
particle are unsuitable for these cladocerans due to a lack of being trapped
and an inability to ingest respectively.
A suitable upper limit of algae size consumed was determined to be
approximately 35 μm with algae 15 μm in length were most abundant in the diet (Fileto et al.,
2004). Similar results were determined
by Pagano (2008), though algal abundance in the diet (based on size) differed
and was varied. Life History/
Growth and Reproduction: Moina micrura individuals, like other cladocerans, molt in order to allow
for body growth (Balcer et al., 1984).
As the old shell is removed, water is taken in to increase body volume
before the new exoskeleton hardens.
Molting occurs many times throughout the life span of M. micrura. M.
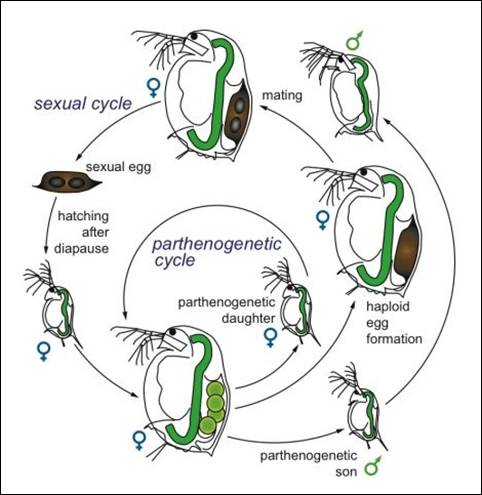
micrura exhibits cyclic parthenogenetic reproduction in which during
favorable conditions, adult females produce unfertilized eggs which are
deposited into the brood chamber following a molt. These eggs develop into juvenile females
which are released from the brood chamber at the next molt. The free swimming juveniles then molt and
grow several times, ultimately reaching adulthood. When conditions become adverse, a female
will produce special eggs that develop into males. Once these males reach maturity, the
females will produce haploid eggs which are then fertilized by the
males. The eggs are then released when
the female molts and are encased in the carapace. This complex is called an ephippium and is
resistant to adverse conditions. Once
conditions are favorable, the fertilized eggs hatch to release
parthenogenetic female offspring (Balcer et al., 1984; Martínez-Jerónimo et
al., 2007). Figure 6 shows this as
generalized cladoceran lifecycle.
Adult longevity is approximately 12 days in the wild and reproductive
peak occurs between five and ten days (Jana and Pal, 1985). Adverse
conditions leading to sexual reproduction are varied and include zooplankton
density, temperature, adequate food quality and quantity and photoperiod
among others (Martínez-Jerónimo et al., 2007). Crosetti and Margaritora (1987) determined
that M. micrura populations in Castelporziano
Park, Italy were most prevalent between May and August, and that sexual
reproduction was most likely induced by temperature and photoperiod, as well
as competition. Martínez-Jerónimo et
al. (2007) determined that volume of the container used in lab experiments of
cultured M. micrura was a
significant inducer of sexual reproduction.
Jana and Pal (1985) determined that varying nutrient cultures (food
quality) had a large impact on growth and reproduction of M. micrura. They determined that while egg incubation
was relatively similar across media, longevity, number of parthenogenetic
females produced by a single female, and peak reproduction days varied
greatly (Jana and Pal, 1985). |
Figure
2. M. micrura head, indicating the
ventral sloping as well as the large compound eye.
Figure
3. Post-abdominal claw still within the body.
Figure
4. Post-abdominal claw dissected from body to show detail.
Figure
5. Posterior end of M. micrura exhibiting
the post abdominal setae.
Figure
6. Generalized cladoceran life cycle (Ebert, 2005). |
Literature Cited:
Balcer, M.D., N.L. Korda and S.I. Dodson. 1984. Zooplankton of the Great Lakes: A Guide to the Identification and Ecology of the Common Crustacean Species. The University of Wisconsin Press. Madison, Wisconsin. Crosetti, D. and F.G. Margaritora. 1987. Distribution and life cycles of cladocerans in temporary pools from central Italy. Freshwater Biology. 18:165-175 Ebert, D. 2005. Introduction to Daphnia Biology. (http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=daph&part=ch2) Fileto, C., M.S. Arcifa, A.S. Ferrão-Filho and L.H.S. Silva. 2004. Influence of phytoplankton fractions on growth and reproduction of tropical cladocerans. Aquatic Ecology. 38:503-514 Fileto, C., M.S. Arcifa, R. Henry and R.A.R. Ferriera. 2010. Effects of temperature, sestonic algae features, and seston mineral content on cladocerans of a tropical lake. International Journal of Limnology. 36:135-147. Hart, R.C. 1990. Zooplankton distribution in relation to turbidity and related environmental gradients in a large subtropical reservoir: patterns and implications. Freshwater Biology. 24:241-263. Jana, B.B. and G.P. Pal. 1985. Life history parameters of Moina micrura (KURZ.) grown in different culturing media. Water Research. 19:863-867 Martínez-Jerónimo, F., J. Rodríguez-Estrada, R. Villaseñor-Córdova. 2007. Effect of culture density and volume on Moina micrura(Kurz, 1874) reproduction, and sex ratio in the progeny. Hydrobiologia. 594:69-73 Pagano, M. 2008. Feeding of tropical cladocerans (Moina micrura, Diaphanosoma
excisum) and rotifer (Brachionus
calyciflorus) on natural phytoplankton: effect of phytoplankton
size–structure. Journal of Plankton Research. 30:401-414 |
|