|
Site created by: W. Cody Webster |
|
|
Sida Crystallina Taxonomy Kingdom - Animalia Sida
crystalline has
three subspecies: Sida crystallina
crystalline, Sida crystallina ortiva, and Sida crystallina Anatomy Sida crystallina
is a larger species of the Cladocerans. Females can
reach up to 4.0 mm in length and males can reach up to 2.0 mm in length (Korovchinsky, 1992; Balcer et
al. 1984). Like others in the Sididae family, S. crystalline have a bivalve, oblong
carapace that covers 6 thoracic appendages, a large head without a headshield, and large moveable antenules
(Korovchinsky, 1992; Balcer
et al. 1984). Similar to other Clodocerans, the
first pair of antennae (antennules) have 9 sensory papillae which are used
for chemosensory purposes. The second pair of antennae (antennae) are used for swimming (Figure 1; Thorp & Covich 2001; Korovchinsky,
1992). These have setae (>14)
arranged in a row on the dorsal branch of the 2nd antennae (Figure
3; Balcer et al. 1984). Male’s express much larger antennules than females do. S. crystallina is identifiable from other members of the
Sida genera by its dorsal antennae being 3
segmented (Figure 3; Pennak, 1978). Another
distinguishable characteristic, present within the Sida
genera, are their anchoring organs (maxillary gland), used to attach to
substrate, that secrete a gelatinous glue (Thorp & Covich
2001). This is visible on the dorsal
part of their body behind their blunt head (Figure 1). Distribution Sida
crystallina is a plant-associated species found in the litoral part of lakes, reservoirs and rivers (Korovchinsky, 1992). They are a less abundant crustacean
in the Laurentian Great Lakes that exhibit a patchy distribution pattern
(Cerbin1 et al. 2003). Patches range from low densities to densities reaching
370-5000 organisms m-3. They have been
reported in Lake Superior, Lake Huron, Feeding Ecology Sida
crystallina are sessile filter feeders (Cerbin1 et al. 2003).
However, unlike most plant-associated species they primarily feed on
phytoplankton (Fairchild, 1982). S. crystallina has a relatively fast metabolism rate and
is comparably higher than other Clodocerans. A
study done by Downing and Peters (1980) showed that filtering rates decreased
while food concentration increased. An increase in respiration occurs when
there is a high abundance of food. This is due most likely by the increased
metabolic needs in order support the increased grooming of the filters on the
thoracic appendages. Interestingly, this may lead the animal to starvation by
not meeting the increased metabolism demands (Thorp & Covich
2001). Feeding for S. crystalline typically takes place during the night.
However, they will feed during the day. Fairchild (1982) found that feeding
densities increase by one-third at night which is consistent with most plant
associated zooplankton. Reproduction Sida
crystallina can reproduce sexually and asexually, which is
dependent on environmental conditions. However, the primary mode of
reproduction is parthenogenetically. The presence
of a certain stimuli such as crowding, photoperiod, or food, is when they
will switch to sexual reproduction. The
reproductive cycle generally begins around March or April, depending on
environmental cues. According to Green (1966) egg production seems to be high
during the spring and fall months with a low during the summer months. In
October the majority of females will transition into producing resting eggs,
which will not hatch until the following spring (Green, 1966). This change affects the structure of the
population in November and December. At this time the females grow to a large
size due to few recruits in the autumn population.
|
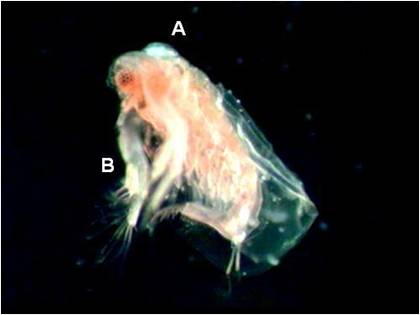
Figure 1 Sida crystallina’s
dorsal maxillary gland (A) and its second antennules (B).
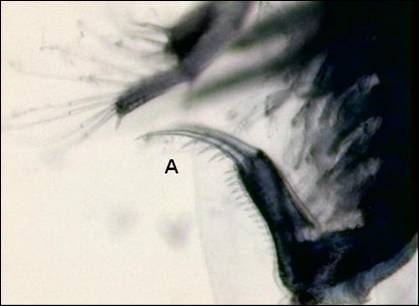
Figure 2. Post abdominal claw with setae
(A).
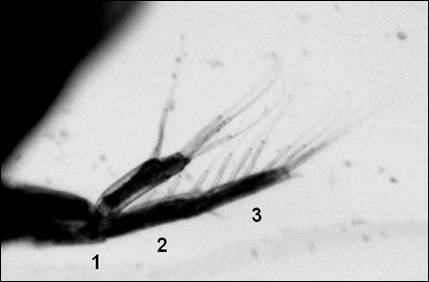
Figure
3.
Ventral and dorsal ramus of second antennae with swimming
hairs. Dorsal ramus showing 3 segments.
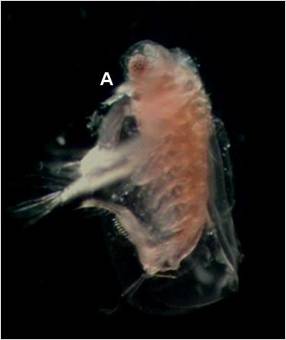
Figure 4. Sida crystallina with rostrum (A). |
Works Cited:Balcer, M.D., N.L. Korda,
and S. I. Dodson. (1984) Zooplankton of the Great Lakes: A Guide to the
Identification and Ecology of the Common Crustacean Species. Pp110. Cerbin,. S., D. J. Balayla,
and W. J. Van de Bund. (2003) Small-scale
distribution and diel vertical migration of zooplankton
in a shallow lake (Lake Naardermeer, the
Netherlands). Hydrobiologia, 491: 111–117. Downing, J.
A., and R. H. Peters. (1980) The Effect of Body Size and Food Concentration
on the in Situ Filtering Rate of Sida crystallina. Limnology and Oceanography, 25:
883-895. Fairlchild, G. W. (1980) Movement and Microdistribution
of Sida crystallina
and Other Littoral Microcrustacea. Journal
of Ecology, 62: 1341-1352.
Green, J. (1966) Seasonal Variation in Egg
Production by Cladocera. Journal of Animal Ecology, 35: 77-104.
Korovchinsky, N. M. (1992) Guides to the
Identification of Microinvertebrates of the
Continental Waters of the World 3: Sididae
& Holopediidae: (Crustacea:
Daphniiformes), pp. 1-5. SPB Academic Publishing.
the Hague, the Netherlands. Pennak, R. W. (1978) Fresh-Water Invertebrates of the Sons, New York, New York. Thorp, J.
H., and A. P. Covich. (2001) Ecology and
Classification of North American Freshwater Invertebrates, pp. 850-872. Second Edition. Academic
Press. |
|