|
Site
created by: Briana Skufca Organism: Daphnia parvula
|
|
|
Kingdom -
Animalia Phylum - Arthropoda Subphylum - Crustacea Class - Branchiopoda Order - Diplostraca Suborder - Cladocera Family - Daphniidae Genus - Daphnia Species - Daphnia parvula (Myers et
al., 2016)
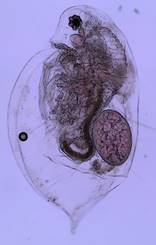
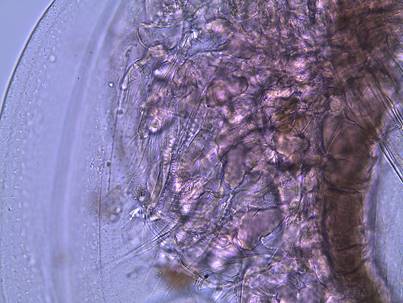
Figure 1: An adult female Daphnia
parvula Systematics Systematic
studies of the genus daphnia are primitive. There are over 100 species within
the genus Daphnia and current
research shows that 33 of these species are present in North America.
Identification of Daphnia species
is revealed through morphological differences which may be difficult to see (Colbourne
& Hebert 1996). Anatomy Cladoceran
are characterized by
their body being enclosed in a bivalve carapace and they have five appendages
adapted for gas exchange and filtering. The head contains a large, dark
compound eye (Balcer et al., 1984). D. parvula can
be identified by the absence of an ocellus and a small and concave head
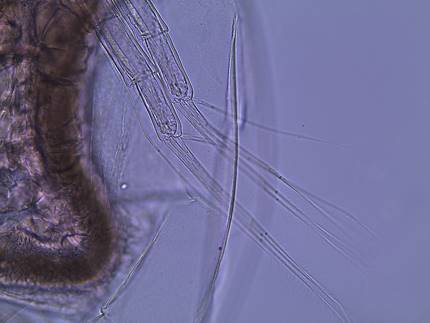
(figure 3). The first antennae are attached to the ventral side of the head
and they have small setae (figure 3). The second antennae are large and are
used for swimming (figure 4). A small rostrum projects from the head (Balcer et al., 1984). D.
parvula have a widely rounded head and their
posterior spine is less than one-quarter of the valve length (Ward et al.,
1959) (figure 5). D. parvula contain a posterior claw (figure 6).
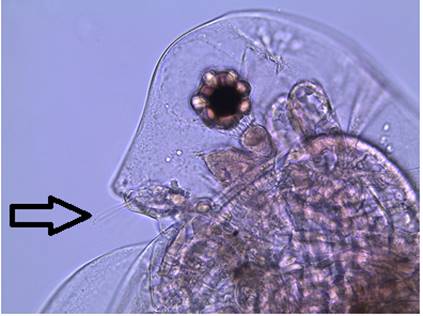
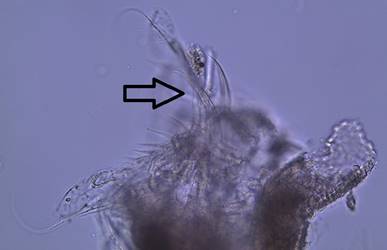
Figure
3: A close up of the head of a female D.
parvula. Note the large, dark compound eye,
concave head, and the absence of an ocellus. The arrow points out the first
antennae.
Figure
4: The second antennae of D. parvula. This appendage is used for swimming.
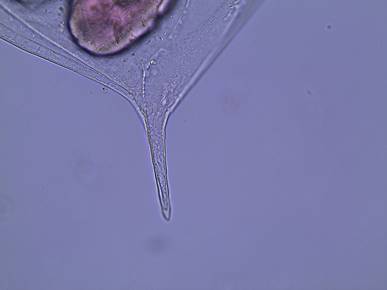
Figure
5: The posterior spine of D. parvula.
Figure 6: The claw of a dissected D. parvula. Geographic Distribution and Vertical
Migration Daphnia generally reside in the open-water
zone of lakes and oceans (Peņalva-Arana et al., 2007, Winder & Mooij,
2004). Daphnia are a very common
species and they are found throughout the Americas, Europe, and Australia.
There are 33 known species of daphnia in North America (figure 7), this
continent has the greatest species richness of daphniids
(Colbourne & Hebert 1996). Both juvenile
and adult Daphnia are daily diel
vertical migrators. Like many cladoceran, daphniids avoid the surface during daylight and migrate
up the water column at night (Rose et al., 2012). Research suggests that the
migrations are a mechanism to avoid predation by planktivorous
fish during the day (Zaret & Suffern 1976). It is confirmed that avoiding fish is an
important factor however, UV exposure has been shown to be a more important
driver of Daphnia vertical
migration. Damage received from UV exposure will elicit a more significant
downward migration than when Daphnia are
in the presence of fish alone (Rose et al., 2012). D. parvula reach maximum density in the fall and
remain high in numbers through spring (Taipale et al., 2009,
Pace et al., 1984). Population densities can be compared to biotic and abiotic
factors in the water column. Densities are high in winter when algal biomass
is lower and populations decrease in warmer months when algal biomass and
temperature increases. Populations are also affected by the increased
population of D. parvula
predator Chaoborus (Pace et al., 1984).
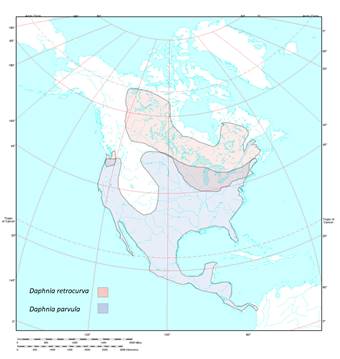
Figure 7: This map shows the North
American distribution of D. retrocurva and D.
parvula. D. parvula is
shown in blue and is widely distributed throughout North America (Constanzo & Taylor, 2010). |
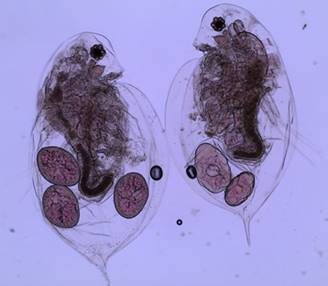
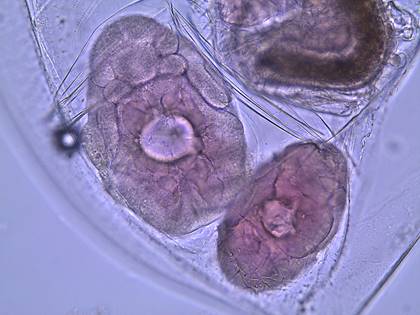
Figure 2: Two adult female daphnia with eggs in their brood
pouches. Feeding Ecology D. parvula is an herbivorous species which consumes
through filter feeding (Peņalva-Arana et al., 2007, Balcer et al., 1984).
Within their carapace, Daphnia contain
four ventral thoracic feeding appendages (figure 8, 9) which create a feeding
current to filter the water for food particles nonstop starting at birth at a
rate of all algae in 4ml of water in one hour (Peņalva-Arana et al., 2007). Their
diet consists of mainly phytoplankton and methane-oxidizing bacteria (Taipale
et al., 2009). Daphnia biomass is
highest in autumn and research suggests that this is because mixing during
this season results in methanotrophic bacteria
which can sustain high populations of Daphnia
(Taipale et al., 2009).
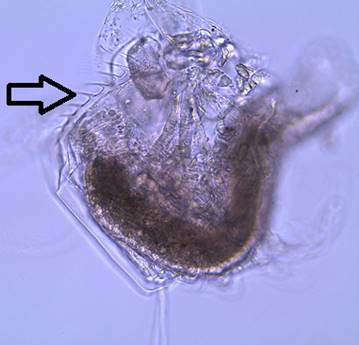
Figure
8: The gut of a dissected D. parvula. The arrow is showing the filtering teeth.
Figure
9: A picture of the filtering teeth in an intact D. parvula. Life History Daphnia growth is highly dependent on the environment.
Availability and quality of food as well as abiotic factors such as
temperature and pH are factors that determine Daphnia growth. Some studies suggest that primarily phosphorus in
the environment assists with Daphnia growth
and survival (Acharya et al.,
2004), other studies suggest that nitrogen and carbon influence growth (Mueller-Navarra, 1995). A study of Daphnia growth done by Lampert & Trubetskova showed that ultimately, concentration of food
has the greatest influence on growth (1996). In order to grow, Daphnia
must molt their exoskeleton. After each molt, they take in water to
rapidly increase their volume before their new molt hardens. They typically
molt two to five times to reach maturity and they can molt up to 25 times
after (Balcer et al., 1984). For the
majority of the year, Daphnia reproduce
through cyclic parthenogenesis using mitosis. During favorable conditions,
the mother Daphnia deposits 2 to 20
2N eggs in her brood pouch (figure
2, 10, 11) and the juveniles are released during the next maternal molt
cycle. These juveniles are identical to the mother (Balcer
et al., 1984, Fink et al., 2011). When environmental conditions become
unfavorable, Daphnia produce
resting eggs covered by an ephippium, a protective
covering, which undergo diapause. This switch is caused by three
environmental stimuli including food limitation (starvation), crowding, and
the amount of illumination received (length of day) (Kleiven
et al., 1992). Resting egg production requires meiosis and a male Daphnia. During these stressful times,
females produce haploid (N) eggs
and diploid (2N) eggs which become
males. The diploid male Daphnia produce
haploid sperm which fertilize the haploid eggs. These now diploid eggs will
become a fertilized resting egg and when favorable conditions return, they
will hatch into females. This process results in genetic recombination and
helps maintain diversity in the population.
Figure
10: Eggs in the brood pouch of a pregnant D.
parvula.
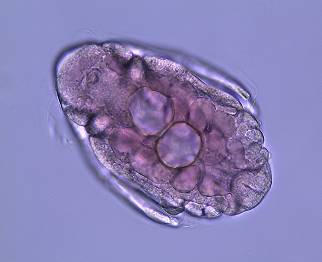
Figure
11: A D. parvula
egg. |
|
Works
Cited:
Acharya, K., Kyle, M., & Elser, J. J. (2004). Biological stoichiometry of Daphnia growth: an ecophysiological test of the growth rate hypothesis.Limnology and Oceanography, 49(3), 656-665. Balcer,
M. D., Korda, N. L., & Dodson, S. I. (1984). Zooplankton
of the Great Lakes: A guide to the identification and ecology of the common
crustacean species. Madison, WI: University of Wisconsin Press. Colbourne, J. K., & Hebert, P. D. (1996). The systematics of North American Daphnia (Crustacea: Anomopoda): a molecular phylogenetic approach.Philosophical Transactions of the Royal Society of London B: Biological Sciences, 351(1337), 349-360. Costanzo, K. S., & Taylor, D. J. (2010). Rapid ecological isolation and intermediate genetic divergence in lacustrine cyclic parthenogens. BMC evolutionary biology, 10(1), 1. Fink, P., Pflitsch, C., &
Marin, K. (2011). Dietary essential amino acids affect the reproduction of
the keystone herbivore Daphnia pulex. PLoS
One,6(12), e28498. Kleiven, O. T., Larsson, P., & Hobæk, A. (1992). Sexual reproduction in Daphnia magna requires three stimuli. Oikos, 197-206. Lampert, W., & Trubetskova, I. (1996). Juvenile growth rate as a measure of fitness in Daphnia. Functional Ecology, 631-635. Mueller-Navarra, D. (1995). Evidence that a highly
unsaturated fatty acid limits Daphnia growth in nature. Archiv
fur Hydrobiologie, 132, 297-297. Myers, P., R. Espinosa, C. S. Parr, T. Jones, G. S.
Hammond, and T. A. Dewey. 2016. The Animal Diversity Web (online). Accessed
at http://animaldiversity.org. Pace, M. L., Porter, K., & Feig, Y. S. (1984). Life history
variation within a parthenogenetic population of Daphnia parvula
(Crustacea: Cladocera).Oecologia, 63(1), 43-51. Peņalva-Arana, D. C., Moore, P. A., Feinberg, B. A., DeWall, J., & Strickler, J. R. (2007). Studying Daphnia feeding behavior as a black box: a novel electrochemical approach. Hydrobiologia, 594(1), 153-163. Rose, K. C., Williamson, C. E., Fischer, J. M., Connelly, S. J., Olson, M., Tucker, A. J., & Noe, D. A. (2012). The role of ultraviolet radiation and fish in regulating the vertical distribution of Daphnia. Limnology and Oceanography,57(6), 1867. Taipale, S., Kankaala, P., HÄMÄLÄINEN, H., & Jones, R. I. (2009). Seasonal shifts in the diet of lake zooplankton revealed by phospholipid fatty acid analysis. Freshwater Biology, 54(1), 90-104. Ward, H. B., Whipple, G. C., & Edmondson, W. T. (1959).
Freshwater Biology. New York:
Wiley. Winder, M., Spaak, P., & Mooij, W. M. (2004). TRADE‐OFFS IN DAPHNIA HABITAT SELECTION. Ecology, 85(7), 2027-2036. Zaret, T. M., & Suffern, J.
S. (1976). Vertical migration in zooplankton as a predator avoidance
mechanism. Limnology
and oceanography, 21(6), 804-813. |
||