
Alzheimer’s disease is a progressive, ultimately fatal disease that causes specific brain cells called neurons, to degenerate and die for largely unknown reasons. Patients suffering from Alzheimer’s disease have significantly impaired memory function followed by a gradual decline in judgment, reasoning ability, and verbal skills. Alzheimer’s disease belongs to a group of related neurodegenerative disorders known as “tauopathies”, whose common pathological feature involves a protein named tau. Tau proteins are highly abundant in neurons and are less common in other cell types of the body. Under normal circumstances, tau physically interacts with proteins called microtubules to help cells maintain their shape and transport critical substances to cellular locations where they are needed. However under disease conditions, tau is found to be coated in phosphate molecules, has an altered molecular conformation, and is hypothesized to be broken down into smaller fragments.
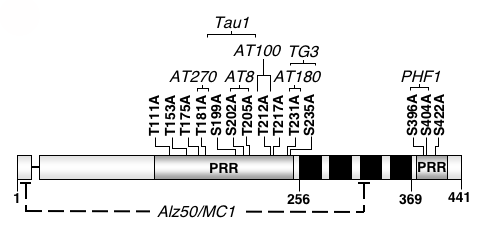
Although scientists are not certain what causes neurons to die in Alzheimer’s disease patients, current research efforts are directed at understanding the molecular pathways leading to neuronal cell death in order to design better ways to treat, or even prevent disease. Research in the Steinhilb laboratory focuses primarily on how the abnormal breakdown of tau and disease-associated tau phosphorylation contribute to cell death. In order to study the role of tau in disease, we take advantage of the powerful tools available in the fruit fly Drosophila melanogaster as a model system. Dr. Mel Feany developed a tauopathy model in Drosophila that is useful for studying the molecular, genetic, and biochemical aspects of tau toxicity in a simple model organism. Transgenic flies expressing human tau recapitulate many features of human Alzheimer’s disease including adult onset, progressive neurodegeneration, early death, and accumulation of hyperphosphorylated tau. The ultimate goal of this research is to understand the underlying causes of tau toxicity to extend healthy life and reduce the burdens of illness and disability. Elucidating how tau phosphorylation and breakdown influence toxicity may lead to new treatment strategies.
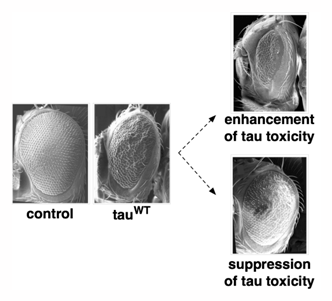
The external appearance of the eye of the fruit fly, Drosophila melanogaster. Scanning electron microscopy (SEM) shows the normal appearance of a non-transgenic fly (control), compared to the moderately rough eye produced by expression of human tau (tauWT). Compared to the eye of tauWT flies, exacerbated roughness is observed under conditions of enhancement of tau toxicity. Conversely, suppression of tau toxicity causes the eye to look more like that of control flies. Gene products such as proteins and enzymes that modify the appearance of the tau rough eye phenotype are referred to as enhancers or suppressors.