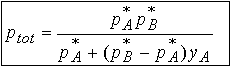Activities
7.6 The solvent activity
General form of the chemical potential for a real or ideal solvent
is given by modification of the equation for the chemical potential of a
mixture.

For an ideal solution, Raoult’s law applies

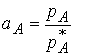
The quantity aA is the Activity of A, an “effective mole fraction” just as fugacity is an effective pressure. Since the chemical potential in terms of pressures is true for both real and ideal solutions we conclude that
![]()
A convenient way of expressing this convergence is to introduce
the activity coefficient, g ,
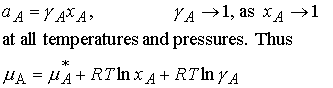
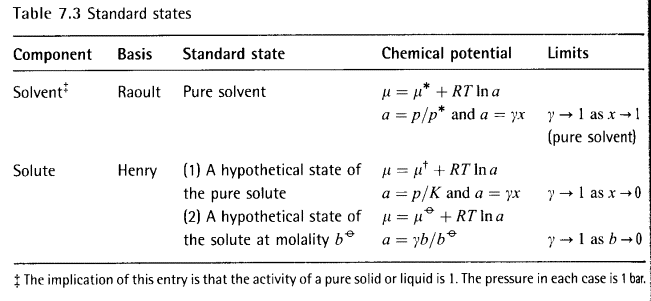
7.7 The solute activity
The problem with defining activity coefficients and standard
states for solutes is that they approach ideal dilute (Henry’s Law) behavior as
![]()
(a) ideal-dilute solutions
For a solute which obeys Henry’s Law, pB = KBxB
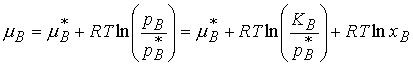
Both KB and pB* are characteristic of the
solvent, so the second term may be combined with the first to give a new
standard chemical potential
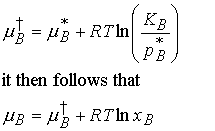
(b) Real solutes
For real deviations from Henry’s law we introduce aB into the
equation above.
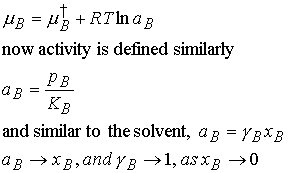
at all temperatures and pressures. Deviations of the solute from ideality disappear as zero
concentration is approached.
Example 7.6 Measuring activity
Use the data in example 7.3 to calculate activity and activity
coefficient of chloroform in acetone at 25 0C, treating it
first as a solvent then as a solute.
Method: For the activity of chloroform as a solvent, form a=p/p* and g = a/x. For activity as a solute, form a = p/K and g = a/x.
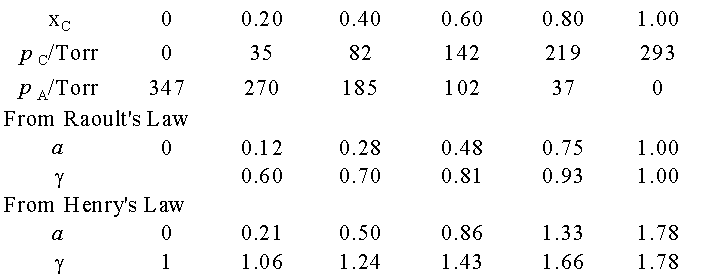
Answer: Because p* = 293 Torr and K
= 165 Torr (previous problem), we construct the following table with x the mole
fraction of chloroform.
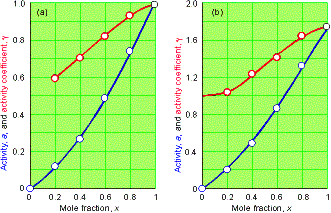
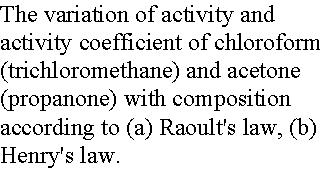
Notice
that g ® 1 as x ®
0 in the Raoult’s Law case;
g ®
0 as x ® 0 in the Henry’s Law case.
(c) Activities in terms of molalities
Another definition of activity. In dilute solutions, to a good approximation, xB » nB/nA because nB is proportional to molality, b, we can write
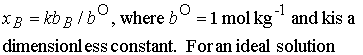
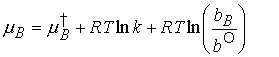
To simplify notation, we interpret b as a relative molality
(replace b/b° by b; in practice, using for b its numerical
value in moles per kg). Then we write
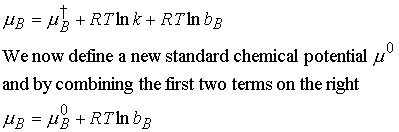
According to this definition, the chemical potential of solute has
its standard value m ° when molality of
B is equal to b° (at 1
mol/kg). Note as b° goes to 0, m B goes to ¥; that is the solution becomes
diluted, so the solute becomes increasingly stabilized. The consequence of this result is that it is
very difficult to remove the last traces of solute from a solution.
Now as before we incorporate deviations from ideality by introducing a
dimensionless activity aB and a dimensionless activity
coefficient gB.
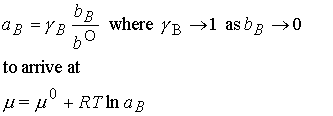
Chapter 8 Phase Diagrams
We will review a systematic way to describe the physical changes
mixtures undergo when they are heated or cooled and when their compositions are
changed.
All phase diagrams can be discussed in terms of a relationship,
the phase rule, derived by J. W. Gibbs.
8.1 Definitions
Phase: A state of matter that is uniform throughout in chemical and
physical composition. (solid, liquid,
gas, solution of two miscible liquids, etc.).
The number of phases in a system is denoted P. A solution of water and acetone has one
phase, P = 1, since they are uniformly mixed. A slurry of ice and water is a
two-phase system, P = 2. A system in
which calcium carbonate undergoes thermal decomposition consists of two solid
phases (calcium carbonate and calcium oxide are not uniformly mixed) and one
gas phase (carbon dioxide).
An alloy of two metals is a two-phase system (P = 2) if the metals
are immiscible, but a single phase system (P = 1) if the metals are miscible.
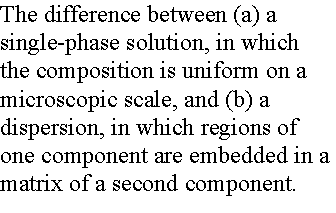
A dispersion is uniform on a macroscopic scale but not on a
microscopic scale, for it consists of grains or droplets of one substance in a
matrix of the other. Such dispersions
are important in materials where the microstructure is fixed to tailor
important properties.
By a constituent of a system we mean a chemical species
(ion or molecule) that is present. A
mix of water and ethanol has two constituents.
A Component is a chemically independent constituent of a
system. The number of components, C, in
a system is the minimum number of independent species necessary to define the
composition of all phases present in the system.
When no reaction takes place, the number of components is equal to
the number of constituents, chemical species.
The mixture of ethanol and water is a two-component system (C = 2).
Consider the phase equilibrium
![]()
3 Phases - To specify the composition of the gas phase we need the
species CO2, and to specify phase two, we need the species CaO. We do not need an additional species to specify
the composition of phase 3 because its identity can be expressed in terms of
the other two constituents by making use of the stoichiometry of the
reaction. Hence the system has 3
constituents but only two components (C = 2).
In general the number of constituents minus the number of chemical
reactions equal the number of components.
Examples: How many
components?
a)
aqueous acetic acid – CH3CO2H and H2O;
C = 2
b)
MgCO3(s) in
equilibrium with its decomposition products.
MgCO3(s) ® MgO(s) + CO2(g)
3 constituents - 1 reaction – C = 2
c)
water (including its ionization)
H2O(l) ® H+(aq)
+ OH-(aq) Looks like 3
constituents, 1 reaction. BUT H and OH
are formed in equal molar amounts.
Really only need the concentration of one and the other is the
same.
2 constituents - 1 reaction – C = 1
d)
NH4Cl(s) ® NH3(g) + HCl(g) in equilibrium
Again 2 constituents – 1 reaction – C=1
e)
Reaction above with additional HCl(s) present.
Concentrations are not related by stoiciometry.
3 constituents - 1 reaction – C = 2
The Variance, F, of a system is the number of intensive
variables that can be changed independently without disturbing the number of
phases in equilibrium.
In a single component, single phase system (C = 1, P =1), the
pressure and temperature may be changed independently without changing the
number of phases, so F = 2. This system
is bivariant and has two degrees of freedom.
If two phases are in equilibrium (ie - liq and vap) in a one
component system such as H2O(l) and H2O(g) (C = 1, P = 2), the T or p can be changed
at will, but the change in one demands an accompanying change in the other to
preserve the number of phases in equilibrium. The system variance in this case
is one (F = 1) one degree of freedom.
.
8.2 The Phase Rule
In one of the most elegant calculations of the whole of
thermodynamics, J. W. Gibbs deduced the phase rule, which is a general
relation between the variance, F, the number of components, C, and the number
of phases at equilibrium, P, for a system of any composition:
![]()
a)
One-component system
For a one-component system, such as pure water, F = 3 - P. When only one phase is present, F = 2 and
both p and T can be varied independently without changing the number of
phases. In other words, a single
phase is represented by an area on a phase diagram. When two phases are in equilibrium, F = 1,
which implies that pressure is not freely variable if temperature is set.
At a given temperature, a liquid has a characteristic vapor pressure (F = 1). Thus, the equilibrium of two phases is represented by a line on the phase diagram.
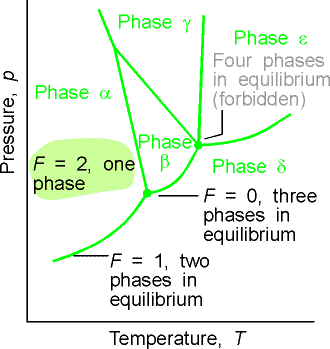
When the three phase are in
equilibrium, F = 0 and the system is invariant. This special condition can be established only at a definite
temperature and pressure that is characteristic of the substance and
outside our control. The equilibrium of
three phase is represented by a point (the triple point) on the phase
diagram.

8.3 Vapor pressure diagrams
The vapor pressure of the components of an ideal solution of two
volatile liquids are related to composition according to Raoult’s law:
![]()
The total pressure ptot of the mixture is
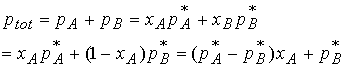
The total pressure is a linear function of composition of the
ideal solution.
a) The composition of vapor
The partial pressures of the components are given by Raoult’s
Law. From Dalton’s law we write for the
mole fractions of the vapor, yA
and yB,
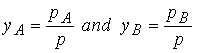
Provided the mixture is ideal, the total pressure can be expressed
in terms of mole fractions in the liquid using Raoult’s Law for pJ and the equation
above for total vapor pressure p, which gives
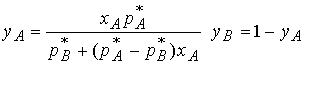
The compostion (mole fraction) in. the vapor, yA, is
related to the composition in the liquid, xA. By solving the equation for xA
and substituting into the equation for ptot we relate the total
vapor pressure to composition of the vapor.